Graphite is a material that’s often associated with pencils, but scientifically, it’s far more fascinating. What makes it even more intriguing is its ability to conduct electricity, which is quite unexpected from a non-metal.
As someone who’s delved into this topic professionally, I’ve always found the conductivity of graphite a compelling topic to explore. Let’s uncover the exact science behind why does graphite conduct electricity so effectively.
What Makes Graphite Able to Conduct Electricity Despite Being a Non-Metal?

At first glance, it seems counterintuitive that graphite, a non-metal and a form of carbon, can conduct electricity.
Most non-metals are known for their inability to allow electric current to pass through them, as their electrons are typically held tightly within atoms or bonds.
However, graphite stands out as an exception due to its distinct atomic structure and electron behaviour. Unlike typical non-metals, graphite’s structure allows certain electrons to move freely.
This is the foundation for its electrical conductivity, and understanding why requires a closer look at the nature of carbon bonding in graphite and how it differs from other non-conductive non-metals.
The Role of Carbon’s Valence Electrons
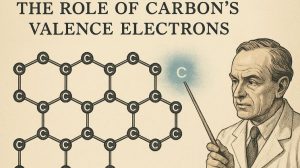
Carbon has four valence electrons.
In graphite:
- Each carbon atom forms three strong covalent bonds with other carbon atoms in the same plane, creating a hexagonal arrangement.
- This sp² hybridisation uses up only three of the four valence electrons.
- The remaining one electron per carbon atom is not involved in bonding and becomes delocalised.
These delocalised electrons are not fixed to any single atom and are free to move across the plane of carbon atoms, a property typically found in metals.
Why This Matters for Conductivity?
Electric current is simply the flow of charged particles, usually electrons.
For a material to conduct electricity:
- It must have mobile charge carriers (such as free or delocalised electrons).
- These carriers must be able to move in response to an electric field.
Graphite meets both criteria. The delocalised electrons serve as charge carriers, and their movement across the hexagonal carbon layers enables efficient electrical conduction.
Comparison with Typical Non-Metals
To better understand graphite’s uniqueness, here’s a table comparing it to a more conventional non-metal:
| Feature | Graphite (Non-metal) | Typical Non-Metal (e.g., Sulphur) |
| Valence electrons involved | 3 in bonds, 1 delocalised | All valence electrons used in bonds |
| Electron mobility | High (due to delocalised electrons) | Very low or none |
| Electrical conductivity | High | Poor/None |
| Bonding type | Covalent with delocalisation | Covalent only |
| Structure | Layered hexagonal lattice | Molecular or simple covalent structure |
Why is Graphite a Good Conductor of Electricity?
Graphite is a good conductor of electricity because of its unique atomic structure and the presence of delocalised electrons.
Each carbon atom in graphite forms three covalent bonds with neighbouring carbon atoms in a flat, hexagonal arrangement. This leaves one electron per carbon atom that is not involved in bonding.
These unbonded electrons become delocalised, meaning they are free to move throughout the layers of the graphite structure.
When a voltage is applied, these electrons can flow across the layers, allowing graphite to conduct electricity efficiently.
Unlike most non-metals, which have tightly bound electrons that restrict conductivity, graphite’s mobile charge carriers enable it to behave more like a metal in electrical applications.
Its layered structure also supports electron movement along the planes, enhancing its conductive properties in that direction.
How Does the Structure of Graphite Allow Electron Movement?
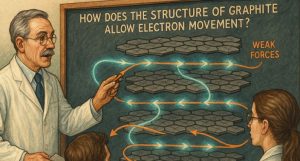
The electrical properties of graphite are deeply rooted in its molecular structure. Each carbon atom in graphite is bonded to three others through strong covalent bonds, forming a flat, two-dimensional network.
These networks stack upon one another to form layers, which are only weakly held together by van der Waals forces.
The fourth valence electron from each carbon atom, which is not involved in bonding, becomes delocalised. These delocalised electrons form an electron cloud that is free to move within the plane of each layer.
Because these electrons are not tied to any specific atom, they can travel across the structure when a voltage is applied, allowing the material to conduct electricity.
This mechanism is remarkably similar to what occurs in metallic bonding, where free electrons drift through a lattice of positive ions.
In graphite, while the atoms themselves are non-metallic, the presence of a delocalised electron system allows for conductivity on par with many metals.
Why Are Delocalised Electrons in Graphite Important for Conductivity?
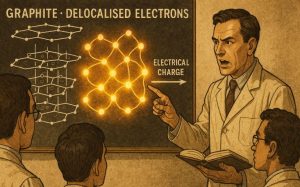
The key to understanding why graphite conducts electricity lies in the concept of delocalisation.
In graphite, each carbon atom contributes one electron to a shared system of electrons that is not associated with any particular atom or bond. These electrons form a ‘sea’ that spans the entire structure of a layer.
Delocalised electrons are able to move freely across the graphite layers. When an electric field is applied, these electrons are pushed in a specific direction, resulting in an electric current.
Without this electron mobility, graphite would behave more like an insulator than a conductor. The electrical conductivity of graphite is thus highly dependent on the ease with which these electrons can move.
In the context of scientific materials, graphite demonstrates that electron delocalisation is not limited to metallic elements but can also occur in specially structured non-metals.
How Does Graphite’s Covalent Bonding Influence Its Electrical Properties?
Graphite’s electrical behaviour is a direct consequence of its sp2 hybridised carbon atoms. Each carbon atom forms three sigma (σ) bonds with adjacent carbon atoms in the same plane.
These bonds create the hexagonal pattern seen in each layer of graphite. The remaining unhybridised p-orbital contains one electron, which is not involved in bonding.
These unpaired electrons from the p-orbitals combine to form π-bonds that are delocalised across the entire layer. This results in a conjugated system, which is a continuous network of overlapping p-orbitals.
The delocalisation of these π-electrons is what allows electrical current to pass through the material.
This combination of strong in-plane covalent bonds and mobile π-electrons ensures both structural integrity and electrical functionality.
It is a clear example of how electronic structure can directly influence macroscopic properties such as conductivity.
In What Way Is Graphite’s Lattice Structure Designed for Conduction?
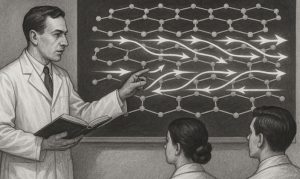
Graphite’s crystalline form is made up of stacked graphene sheets. Within each sheet, the carbon atoms are tightly bonded in a hexagonal arrangement.
However, the layers themselves are loosely connected by weak intermolecular forces. This feature allows them to slide over one another easily, which is why graphite feels slippery to the touch.
From a conductivity standpoint, the significance lies in the movement of electrons within the plane of the layers.
The weak interlayer forces do not support efficient electron movement across layers, which is why graphite conducts electricity predominantly in one direction, along the planes.
The lattice structure provides a balance between stability and flexibility. While the covalent bonds maintain a solid framework, the delocalised electrons within each sheet ensure that the material remains conductive.
| Property | Graphite Description |
| Bonding | Covalent (in layers), van der Waals (between layers) |
| Electron mobility | High (in-plane), Low (perpendicular) |
| Electrical conductivity | Directional (anisotropic) |
| Structural characteristic | Layered hexagonal lattice |
This anisotropic behaviour is an important consideration in applications where directional conductivity is essential.
Why Does Graphite Conduct Electricity but Not Diamond?
Graphite and diamond are both allotropes of carbon, meaning they are made entirely of carbon atoms but arranged in different structures.
This variation in structure leads to vastly different electrical properties, graphite is a good conductor of electricity, whereas diamond is an excellent insulator.
Graphite: A Conductor of Electricity

Graphite conducts electricity due to the presence of delocalised electrons in its structure. Here’s how:
- Each carbon atom in graphite is sp² hybridised, forming three covalent bonds with neighbouring carbon atoms.
- The fourth valence electron is not involved in bonding and becomes delocalised within the plane.
- These delocalised electrons are free to move across the graphite layers.
- When an electric field is applied, these electrons flow, creating an electric current.
This makes graphite an effective conductor, especially along the layers where these electrons can move easily.
Diamond: An Electrical Insulator
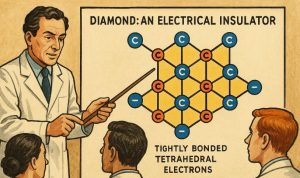
In contrast, diamond does not conduct electricity because of its tightly bound electron configuration:
- Each carbon atom in diamond is sp³ hybridised, forming four covalent bonds in a tetrahedral structure.
- All four valence electrons are used in bonding; no free or delocalised electrons are available.
- As a result, electrons are fixed in place, and there are no charge carriers to support electrical conductivity.
Key Structural Differences at a Glance
| Property | Graphite | Diamond |
| Hybridisation | sp² | sp³ |
| Structure | Layered hexagonal planes | 3D tetrahedral lattice |
| Electron availability | 1 delocalised electron per atom | No delocalised electrons |
| Conductivity | High (within layers) | None (electrical insulator) |
| Bonding | 3 covalent bonds per atom | 4 covalent bonds per atom |
How Does Graphite Compare to Diamond in Terms of Electrical Conductivity?
Graphite and diamond are both composed entirely of carbon atoms, yet their physical and electrical properties are vastly different. This contrast arises from the way the atoms are bonded in each structure.
In diamond, each carbon atom forms four covalent bonds in a tetrahedral configuration. This three-dimensional network uses all four valence electrons from each carbon atom, leaving no free or delocalised electrons.
Consequently, diamond is an electrical insulator, as it lacks the charge carriers needed to conduct electricity.
Graphite, by contrast, uses only three of its four valence electrons for bonding. The fourth electron remains free and forms part of a delocalised system. This structural distinction is why graphite is conductive while diamond is not.
This comparison highlights the critical role of atomic arrangement and electron availability in determining a material’s conductive properties.
Where Is Graphite Used as a Conductor in Real Life?
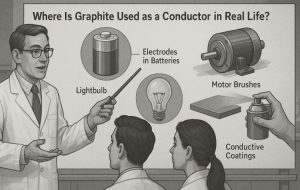
Graphite’s ability to conduct electricity has made it indispensable in various industrial and technological contexts.
Its applications are not limited to simple uses like pencil leads but extend into advanced fields that require reliable and efficient conductors.
Some common uses of conductive graphite include:
- Electrodes in batteries and electrolysis cells, where stable electrical conduction is essential.
- Brushes in electric motors, taking advantage of graphite’s ability to maintain contact and conduct current under friction.
- Conductive coatings in anti-static and electromagnetic shielding applications.
- Touchscreens and sensors, where thin layers of conductive material are needed for responsiveness.
- Crucibles and furnace linings in metallurgy, due to its high thermal resistance in combination with conductivity.
In many of these applications, graphite is valued not only for its conductivity but also for its chemical inertness and thermal stability. Its use is widespread across the automotive, aerospace, chemical, and electronics industries.
What Makes Graphite a Unique Material in Material Science?

In material science, graphite is a subject of extensive study due to its unusual combination of properties. Unlike metals, it is lightweight and corrosion-resistant, yet it shares key electrical characteristics with metallic conductors.
The following attributes make graphite especially valuable:
- High electrical conductivity within layers due to delocalised electrons
- Excellent thermal conductivity and resistance to heat
- Chemically inert and stable even at high temperatures
- Layered structure enabling lubrication and mechanical flexibility
These properties have led to increased interest in graphite-based materials like graphene, which is a single layer of graphite and is even more conductive and stronger than the bulk form.
Research continues into expanding the capabilities of graphite in areas such as energy storage, flexible electronics, and nano-engineering.
Can the Electrical Conductivity of Graphite Be Enhanced?
Yes, the electrical performance of graphite can be enhanced through several techniques. One of the most prominent developments in this area is the isolation of graphene, which is essentially a single atomic layer of graphite.
Graphene exhibits extraordinary conductivity, even higher than traditional graphite, due to its unrestricted electron movement.
Additionally, doping graphite with elements such as nitrogen or boron can alter its electronic properties and increase its conductivity. These processes are used in developing high-performance electrodes for lithium-ion batteries and other energy applications.
Advances in composite materials that combine graphite with polymers or metals are also making it possible to customise their conductivity for specific industrial needs.
With continuing innovation, the potential for using graphite as a high-efficiency conductor is only set to grow.
Conclusion
So, why does graphite conduct electricity? The answer lies in its unique structure, layers of hexagonally arranged carbon atoms with free electrons that can move through the structure.
This arrangement, combined with delocalised electrons, enables graphite to act as an efficient conductor of electricity.
Understanding the science behind graphite helps us appreciate not only its role in everyday items like pencils but also its vast potential in high-end technology and materials engineering.
FAQs
What is the role of free electrons in graphite’s conductivity?
Free or delocalised electrons in graphite act as charge carriers. They move freely across layers, allowing graphite to conduct electricity efficiently.
Why is graphite used in electrodes instead of other materials?
Graphite is chemically stable, conducts electricity well, and withstands high temperatures, all essential for use in batteries and electrolysis.
Does temperature affect graphite’s electrical conductivity?
Yes, like most conductors, graphite’s conductivity changes slightly with temperature. However, it remains conductive over a wide range of conditions.
Is graphite the only non-metal that conducts electricity?
No, other non-metals like carbon nanotubes and graphene also conduct electricity, but graphite is the most widely used and studied.
Can graphite conduct electricity in all directions?
Graphite primarily conducts electricity along the plane of its layers (in-plane conductivity). Perpendicular conduction (through layers) is weaker due to van der Waals forces.
Why is graphite considered a semi-metal sometimes?
Graphite is sometimes classified as a semi-metal because it shares properties with both metals (like conductivity) and non-metals (like lack of luster or ductility).
How is graphite different from graphene in conductivity?
Graphene, a single layer of graphite, exhibits even higher conductivity and strength due to the absence of interlayer resistance found in bulk graphite.






